NT340
Nanosecond laser with high pulse energy and large tuning range
The NT340 enables software-controlled, gapless tuning from 192 nm to 4400 nm with a repetition rate of up to 20 Hz – all from a single device. With a linewidth of less than 5 cm-¹ and a wide range of options, this laser is an excellent choice for a very broad range of spectroscopic applications.

Features
- Software-controlled, seamless
- Wavelength tuning from 192 to 4400 nm
- Up to 90 mJ pulse energy in the visible spectral range
- Up to 15 mJ pulse energy in the UV spectral range
- Up to 20 mJ pulse energy in the MIR spectral range
- 3-5 ns pulse duration
- Up to 20 Hz pulse repetition rate
- Optional separate, common output port for 532/1064 nm beam (separate output port for the 355 nm beam is standard)
- Monitoring the OPO pump laser energy
- Hermetically sealed oscillator cavity protects non-linear crystals from dust and moisture
Applications
- Laser-induced fluorescence (LIF)
- Flash photolysis (flash photolysis)
- Photobiology
- Remote sensing
- Time-resolved spectroscopy
- Nonlinear spectroscopy
- Vibration spectroscopy
- Cavity Ring-Down Spectroscopy (CRDS)
- Cavity Ring-Down Laser Absorption Spectroscopy (CRLAS)
- Infrared spectroscopy (IR spectroscopy)
- Gas spectroscopy
Applications
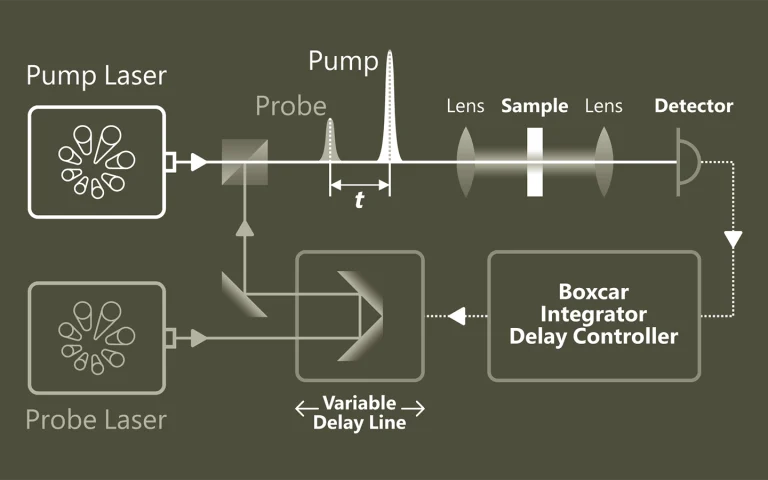
Pumps. Samples. Understanding
Pump-probe spectroscopy provides a deep insight into ultrafast processes at the atomic and molecular level. With the help of pulsed lasers, dynamic changes can be tracked with extremely high temporal resolution. Typical applications are the investigation of charge carrier dynamics in semiconductors, the analysis of electron transfer in chemical reactions and the study of light-induced phase transitions in materials.
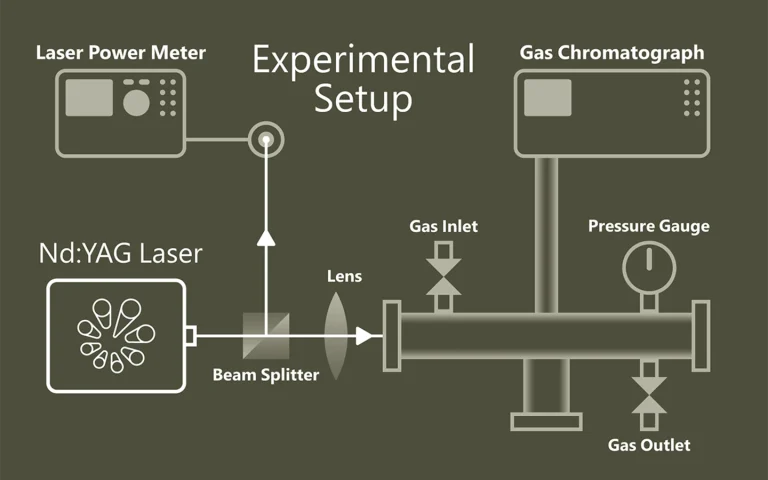
When light separates molecules
Photolysis describes the light-induced splitting of chemical compounds and plays a central role in photosynthesis through water splitting. Laser flash photolysis uses short, intense laser pulses (ns-fs) to generate short-lived intermediates such as radicals or excited states and to observe their temporally resolved absorption. Probe pulses record reaction processes. The use of OPOs significantly expands the possibilities of spectroscopic investigations.
Applications
Scientific publications
Photogeneration and reactions of benzhydrylcations and radicals: A complex sequence ofmechanisms from femtoseconds tomicroseconds
C. F. Sailer, and E. Riedle, Pure and Applied Chemistry 85 (7), 1487-1498 (2013). DOI: 10.1351/PAC-CON-13-04-01.
Benzhydryl radicals and cations are reactive intermediates central to the under-standing of organic reactivity. They can be generated from benzhydryl halides by UV irradi- ation. We performed transient absorption (TA) measurements over the range from femto – seconds to microseconds to unravel the complete reaction scheme. The 290-720-nm probe range allows the unambiguous monitoring of all fragments. The appearance of the radical is delayed to the optical excitation, the onset of the cation signal is found even later. Ab initio calculations show that this non-rate behavior in the 100 fs range is due to wavepacket motionfrom the Franck-Condon region to two distinct conical intersections. The rise of the optical signal with a quasi-exponential time of 300 fs is assigned to the planarization and solvationof the photoproducts. The bond cleavage predominantly generates radical pairs. A subse- quent electron transfer (ET) transforms radical pairs into ion pairs. Due to the broad inter- radical distance distribution and the distance dependence, the ET is strongly non-exponen-tial. Part of the ion pairs recombine geminately. The ET and the recombination are terminated by the depletion of close pairs and diffusional separation. The remaining free radicals and cations undergo further reactions in the nanosecond to microsecond regime.
Luminescence upconversion in colloidal double quantum dots
Z. Deutsch, L. Neeman, and D. Oron, Nature Nanotechnology , 649-653 (2013). DOI: 10.1038/nnano.2013.146.
Luminescence upconversion nanocrystals capable of converting two low-energy photons into a single photon at a higher energy are sought-after for a variety of applications, including bioimaging and photovoltaic light harvesting. Currently available systems, based on rare-earth-doped dielectrics, are limited in both tunability and absorption cross-section. Here we present colloidal double quantum dots as an alternative nanocrystalline upconversion system, combining the stability of an inorganic crystalline structure with the spectral tunability afforded by quantum confinement. By tailoring its composition and morphology, we form a semiconducting nanostructure in which excited electrons are delocalized over the entire structure, but a double potential well is formed for holes. Upconversion occurs by excitation of an electron in the lower energy transition, followed by intraband absorption of the hole, allowing it to cross the barrier to a higher energy state. An overall conversion efficiency of 0.1% per double excitation event is achieved.
Electronic spectroscopy and nanocalorimetry of hydrated magnesium ions [Mg(H2O)n]+, n = 20-70: spontaneous formation of a hydrated electron?
T. Taxer, M. Ončák, E. Barwa, C. van der Linde, and M. K. Beyer, Faraday Discuss. 217, 584-600 (2019). DOI: 10.1039/C8FD00204E. C. Kjær, and S. B. Nielsen, Phys. Chem. Phys. 21, 4600-4605 (2019). DOI: 10.1039/C8CP07340F.
Hydrated singly charged magnesium ions [Mg(H 2O) n] + are thought to consist of an Mg 2+ ion and a hydrated electron for n > 15. this idea is based on mass spectra, which exhibit a transition from [MgOH(H 2O) n-1] + to [Mg(H2O)n]+ around n = 15-22, black-body infrared radiative dissociation, and quantum chemical calculations. Here, we present photodissociation spectra of size-selected [Mg(H2O)n]+ in the range of n = 20-70 measured for photon energies of 1.0-5.0 eV. The spectra exhibit a broad absorption from 1.4 to 3.2 eV, with two local maxima around 1.7-1.8 eV and 2.1-2.5 eV, depending on cluster size. The spectra shift slowly from n = 20 to n = 50, but no significant change is observed for n = 50-70. Quantum chemical modeling of the spectra yields several candidates for the observed absorptions, including five- and six-fold coordinated Mg2+ with a hydrated electron in its immediate vicinity, as well as a solvent-separated Mg2+/e– pair. The photochemical behavior resembles that of the hydrated electron, with barrierless interconversion into the ground state following the excitation.
